| Molecular Sciences Wageningen |

|
| Molecular Sciences Wageningen |

|
| Isolation and characterization of Xylose (Glucose) isomerase from Fervidobacterium gondwanense (1). |
Fervidobacterium gondwanense (F.g.), isolated from heated geothermal waters in Australia is a member of the order of Thermotogales. This bacterium grows between 44 °C and 88 °C, with an optimum at 68 °C. It's ability to grow on xylose indicates the presence of all genes involved in the xylose utilization pathway, including the gene encoding the xylose isomerase enzyme (XylA). This enzyme not only facilitates the conversion of D-xylose into D-xylulose but also the conversion of D-glucose in D-fructose, which is desirable in many industrial processes.The purpose of this research project was to isolate the previously undescribed xylA gene from Fervidobacterium gondwanense. We have produced functionally active F.g. XylA protein in E. coli and biochemically characterized the purified protein. Methods: A mini-library containing F. gondwanense chromosomal DNA was created and screened using a Thermotoga maritima (T.m.) xylA ³²P-ATP DNA probe. A positive restriction fragment of 3kb was used for making a recombinant vector, which was introduced to E. coli cells. The 3kb insert from successfully transformed colonies was sequenced and was found positive for parts of the F.g. xylA gene. Further sequencing of deletion clones made from the recombinant vector and the use of tailed primer PCR revealed the whole F.g. xylA sequence. The acquired F.g. xylA was brought to expression in E. coli strain BL21 (DE3) in parallel with T.m. xylA serving as control. The F.g. XylA protein was isolated, purified and D-glucose isomerization activity was measured. Temperature optimum, pH dependency and metal ion requirements were determined and compared to the enzyme properties of T.m. XylA. The F.g. xylA gene was sequenced and the genes surrounding the F.g. xylA were identified. Comparison of the obtained sequence date to known xylA genes from related Thermotoga species showed up to 59% sequence identity. The deduced amino acid sequence of F. g. xylA was modeled using the known XylA structure of T. neapolitana and both structures were compared concerning the position of lysine residues, which play an important role in the deactivation of the protein under high temperatures and alkaline pH. The XylA from F. gondwanense is a moderately thermophilic enzyme with an optimal activity at 80 °C at slightly alkaline pH. The high amino acid homology with other Thermotoga XylA proteins characterized in earlier research makes this new enzyme an ideal candidate for comparative studies on structure function relationships. F.g. XylA is a suitable enzyme for industrial processes, working at an intermediate temperature where deactivation reactions can be controlled. Further research on thermophilic xylose isomerases is of great value because of their high potential in several industrial processes. They can replace mesophilic enzymes, allowing increased reaction temperatures, thereby increasing yield and reducing costs. Furthermore, the high degree of amino acid homology between different XylA enzymes isolated from these thermophiles provides great opportunities in comparative studies addressing enzyme properties at a molecular level. 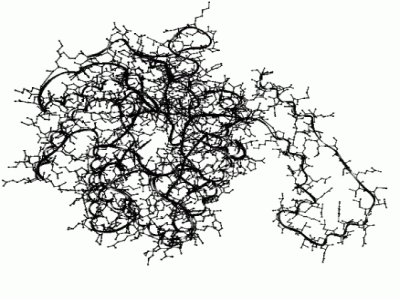 Subunit of F. gondwanense XylA modeled using T. neapolitana XylA as template. |
References:
|
| Activity dependent dynamic changes on PostSynaptic Density of rat brain: A proteomics approach (1). |
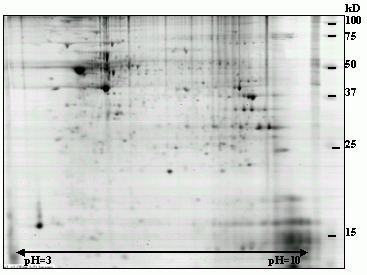 2-DE gel containing purified PSD protein |
References:
|
|
'Indeed, I do not forget that my voice is but one voice, my experience a mere drop in the sea, my knowledge no greater than the visual field in a microscope, my mind's eye a mirror that reflects a corner of the world, and my ideas - a subjective confession'. C.G. Jung. |